Team Led by Scripps Research Scientists Demonstrates Effective New 'Biopsy in a Blood Test' to Detect Cancer
Series of five studies highlights potential of technology to boost patient care, research

Peter Kuhn, PhD, (right) worked with physicians including Kelly Bethel, MD, of Scripps Health to demonstrate the effectiveness of an advanced blood test for detecting and analyzing circulating tumor cells. (Image courtesy of The Scripps Research Institute.)
Scientists from The Scripps Research Institute, Scripps Health and collaborating cancer physicians have successfully demonstrated the effectiveness of an advanced blood test for detecting and analyzing circulating tumor cells (CTCs) — breakaway cells from patients’ solid tumors — from cancer patients. The findings, reported in five new papers, show that the highly sensitive blood analysis provides information that may soon be comparable to that from some types of surgical biopsies.
“It’s a next-generation technology,” said Scripps Research Associate Professor Peter Kuhn, PhD, senior investigator of the new studies and primary inventor of the high-definition blood test. “It significantly boosts our ability to monitor, predict, and understand cancer progression, including metastasis, which is the major cause of death for cancer patients.”
The studies were published February 3, 2012, in the journal Physical Biology.
The new test, called HD-CTC, labels cells in a patient’s blood sample in a way that distinguishes possible CTCs from ordinary red and white blood cells. It then uses a digital microscope and an image-processing algorithm to isolate the suspect cells with sizes and shapes (“morphologies”) unlike those of healthy cells. Just as in a surgical biopsy, a pathologist can examine the images of the suspected CTCs to eliminate false positives and note their morphologies.
Kuhn emphasizes that this basic setup can be easily modified with different cell-labeling and image-processing techniques.
Five new studies, five steps forward
To test the new technology, members of the Kuhn lab at Scripps Research teamed up with pathologists and oncologists at Scripps Health in La Jolla, California; UC San Diego Moores Cancer Center at the University of California, San Diego; the Billings Clinic in Billings, Montana; the Division of Medical Oncology at the University of California, San Francisco; the Center for Applied Molecular Medicine at the University of Southern California, in Los Angeles; and the Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital in Amsterdam, the Netherlands.
The five new studies that resulted from the collaboration not only demonstrate the accuracy and effectiveness of the new test for a number of different cancer types, but also begin to explore the utility of the technology for diagnosing and monitoring patients and improving cancer research in the lab. While other tests for CTCs typically use “enrichment” steps in which suspected CTCs are concentrated—and these methods inadvertently exclude some types of CTCs—the new studies show HD-CTC works well as a no-cell-left-behind process and enables a more complete analysis.
Also striking is the quality of the images. “The high definition method gives a detailed portrait of these elusive cells that are caught in the act of spreading around the body,” said diagnostic pathologist Kelly Bethel, MD, of Scripps Health and Scripps Research, who is the senior clinical investigator on Kuhn’s team. “It’s unprecedented—we’ve never been able to see them routinely and in high definition like this before.”
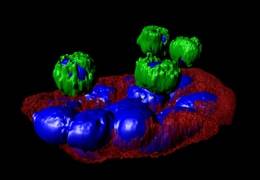
A cluster of lung circulating tumor cells (in red-blue) interacts with normal blood cells (green-blue) recovered from a blood sample of patient with lung cancer. (Image courtesy of the Kuhn lab.)
In the first study, the research team examined 83 advanced cancer patients using HD-CTC to document the test’s sensitivity and accuracy for different cancer types. The scientists found that the test detected five or more CTCs per milliliter of blood in 80 percent of patients with metastatic prostate cancer, 70 percent of those with metastatic breast cancer, 50 percent of those with metastatic pancreatic cancer, and no healthy subjects. The current gold-standard CTC test, known as CellSearch, was notably less sensitive in detecting tumor cells in these samples.
Most patients whose CTC counts surpassed the detection threshold also showed small aggregates of CTCs, which cancer biologists term “microtumor emboli.” These are widely suspected to be incipient metastatic tumors, as well as triggers for the blood clots that often kill advanced cancer patients. In the second study, the scientists showed that HD-CTC could detect these aggregates in 43 percent of 71 patients with advanced prostate, lung, pancreas, and breast cancers, and in none of a group of 15 healthy subjects. “This tells us that HD-CTC could be helpful in studying the origins of cancer metastases and related blood clots, and for predicting them, too,” Kuhn said.
In the third study, the team used HD-CTC to compare circulating tumor cells from prostate cancer patients with cells from prostate cancer cell lines that researchers often use as convenient models for prostate cancer biology in the lab. The team found significant differences between the two classes of cells, in their cell morphology and in the way they were labeled by HD-CTC’s fluorescent tags. “This underscores the need for studying cancer cells from patients, not just model cancer cells that in some ways may be utterly different from the real thing,” Kuhn said.
In the fourth study, the researchers performed HD-CTC tests on 28 patients with advanced non-small-cell lung cancer over periods of up to a year. The team was able to detect CTCs in 68 percent of samples, and found that the numbers of detected CTCs tended to go up as other measures showed cancer progression.
In the fifth and final paper of the series, the team used HD-CTC in 78 patients who had just been diagnosed with various stages of non-small-cell lung cancer. “We demonstrated that we could sensitively detect CTCs even in patients with early-stage cancer,” Kuhn said.
This result points to the possibility of using the HD-CTC blood test not only to evaluate already-diagnosed cancer, but also to help detect cancer in people who are unaware they have it. “If HD-CTC works on the day after cancer diagnosis, as we’ve shown, then one can easily imagine that it would work the day before diagnosis, too,” Kuhn said.
Kuhn and his colleagues now intend to study the use of HD-CTC as a potential screening test and to develop it further for use in clinical monitoring and cancer research. Kuhn has founded a San Diego-based biotechnology company, Epic Sciences, Inc., to develop HD-CTC commercially for companion diagnostic products in personalized cancer care.
Dena Marrinucci, PhD, of Scripps Research was first author of the study, “Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancer”; Edward H. Cho, PhD, of Scripps Research was first author of “Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors”; Daniel C. Lazar of Scripps Research was first author of “Cytometric comparisons between circulating tumor cells from prostate cancer patients and the prostate-tumor-derived LNCaP cell Line”; Jorge Nieva, MD, of Scripps Research was first author of “High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis; and Marco Wendel, PhD, of Scripps Research and Lyudmila Bazhenova, MD, of UC San Diego Moores Cancer Center were first authors of “Fluid biopsy for circulating tumor cell identification in patients with early and late stage non-small cell lung cancer; a glimpse into lung cancer biology.” For more information on the papers, see http://iopscience.iop.org/1478-3975/.
Kuhn’s laboratory is supported by the National Cancer Institute (NCI) of the US National Institutes of Health as the NCI Scripps Physics Oncology Center, which was initially supported through the American Recovery and Reinvestment Act.
About The Scripps Research Institute
The Scripps Research Institute is one of the world’s largest independent, non-profit biomedical research organizations. Scripps Research is internationally recognized for its discoveries in immunology, molecular and cellular biology, chemistry, neuroscience, and vaccine development, as well as for its insights into autoimmune, cardiovascular, and infectious disease. Headquartered in La Jolla, California, the institute also includes a campus in Jupiter, Florida.
For more information, see www.scripps.edu.
Media Contact
- Steve Carpowich
- 858-312-0328
- carpowich.stephen@scrippshealth.org